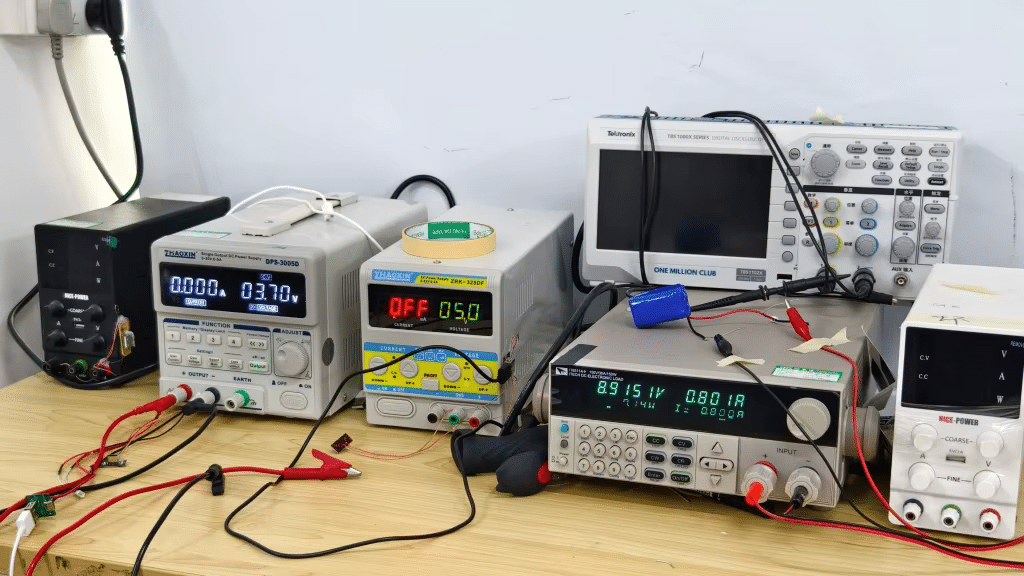
Are you troubled by not knowing the detailed quality control procedures of the upstream factories?
Are you worried about quality issues leading to customer complaints and irreversible brand damage?
For B2B buyers of sex toys, the biggest nightmare is a product safety incident.
One complaint could ruin the reputation you have painstakingly built.
At Luxora, we believe that safety is the foundation of intimacy.
As your “Creative Ally”, we don’t just manufacture products; we manufacture trust.
Let me walk you through how we ensure absolute “safety” from the source to the bedroom.
This article details our safety protocols. For a broader look at our operational excellence, read our Ultimate B2B Guide to Quality Control & Supply Chain.

Table of Contents
ToggleThe First Line of Defense: 100% Body-Safe Raw Materials
Our commitment begins with the materials themselves.
We exclusively use true medical-grade silicone that has passed rigorous biocompatibility testing under FDA standards.
This ensures it is non-toxic, non-allergenic, and will not cause irritation to sensitive human tissue.
But simply claiming to use “medical-grade” material isn’t enough.
We learned this lesson the hard way during a factory audit in 2019—a supplier’s certificate doesn’t always tell the whole story.
To guarantee authenticity and safety, we implemented a mandatory dual-verification system for all raw materials:
-
Supplier-Level Verification: We require comprehensive documentation for every batch of silicone, proving its compliance with FDA standards (U.S. Food and Drug Administration) and its precise chemical composition.
-
In-House Verification: Upon arrival, we conduct our own independent material testing to confirm that the silicone’s composition perfectly matches the specifications. There are no exceptions.
This two-step process ensures that every gram of silicone is traced, tested, and certified at the molecular level. It’s the foundational promise that allows you to represent product safety to your customers with absolute confidence.
👉 Deep Dive: Why material safety is your brand’s best insurance
The Manufacturing Promise: A Rigorous, Multi-Stage Quality Control Process
True quality is forged through systematic checks at every stage of production.
Our comprehensive QC system is designed to identify and eliminate issues long before a product is finished.
This isn’t just a final inspection; it’s a culture of vigilance.
| QC Stage | Purpose | Key Standard & Action |
| Raw Material Inspection | To validate all incoming components. | Every batch is verified against chemical and physical specifications. |
| In-Process Quality Control (IPQC) | To monitor production parameters in real-time. | Our teams constantly check for any deviations during assembly and molding. |
| 100% Functional Inspection | To prevent premature failure and ensure performance. | Every motor is tested for 10,000 operational cycles. Every function is verified. |
| 100% Visual Inspection | To ensure a flawless user experience. | We inspect for any cosmetic defects, surface irregularities, or assembly issues. |
| IPX7 Waterproof Verification | To guarantee durability and safety in use. | Each product must withstand 30-minute submersion at one-meter depth without failure. |
| Pre-Packaging Random Inspection | To provide final statistical confidence. | A final batch sample is rigorously tested before shipment is approved. |
The Final Guarantee: Compliance, Certification & Complete Transparency
Our commitment extends beyond our factory doors.
We provide the documentation and certifications you need to operate smoothly and legally in global markets.
Global Certifications: Your Market Access Shield
Certifications are your legal shield.
Our portfolio demonstrates our commitment to meeting the world’s most stringent safety standards and includes:
-
CE Marking for compliance in the European market.
-
RoHS Certification proving our products meet strict hazardous substance restrictions.
-
FCC Approval for all electronic components sold in North American markets.
Documentation & Traceability: Your Compliance Foundation
Transparency is what separates professional manufacturers from unreliable operations.
Every shipment is accompanied by:
-
Complete technical specification sheets.
-
Material Safety Data Sheets (MSDS).
-
Certified test reports and batch reports showing pass/fail rates.
Our batch management system assigns a unique ID to every production run, creating an unbroken chain of traceability.
When regulatory questions arise, you have immediate access to the data needed to resolve compliance inquiries in hours, not weeks.
O ur Unwavering Commitment to Your Brand’s Safety
ur Unwavering Commitment to Your Brand’s Safety
When you partner with us, you are investing in a comprehensive safety ecosystem that transforms manufacturing uncertainty into marketplace confidence.
Quality shortcuts inevitably lead to higher costs in complaints, recalls, and lost credibility.
Our operation is built to shoulder that quality burden, so you can focus on building relationships and growing your brand.
Frequently Asked Questions (FAQ)
Q1: When sourcing B2B, how can one assess a factory’s quality control capabilities?
A1: Assessing a factory’s quality control capabilities requires a systematic evaluation. We recommend you evaluate from the following key dimensions:
-
System Certifications & Documentation: Check for ISO 9001 and request their QC Plan.
-
Supply Chain Management: Inquire about their raw material supplier audits (e.g., FDA, RoHS reports).
-
In-Process Control: Understand their IQC, IPQC, FQC, and OQC points. Ask about specific testing for motor lifespan and waterproof ratings.
-
Traceability System: Ensure they have a batch management system to quickly resolve issues.
- Transparency: A trustworthy factory welcomes audits and communicates openly.At Luxora, we welcome clients to audit any of these aspects.
Q2: How important is medical-grade silicone for the end-user?
A2: It is the most critical line of defense. Medical-grade silicone is crucial because:
-
Absolute Body Safety (Biocompatibility): It is non-toxic, odorless, and hypoallergenic, preventing irritation even with extended contact.
-
Superior Hygiene: Its non-porous surface makes it easy to clean and prevents bacteria buildup, unlike porous TPE.
-
Brand Reputation: Using inferior materials can lead to health issues and recalls. Medical-grade silicone is a long-term investment in your brand’s value.
Q3: What certification documents can Luxora provide to B2B clients?
A3: We provide a complete set of documents for global compliance:
-
Product Certifications: CE (Europe), RoHS (Hazardous Substances), FCC (USA Electronics).
-
Material Safety: FDA compliance declarations and MSDS (Material Safety Data Sheets).
-
Supporting Documents: Detailed Product Specification Sheets and complete Factory Inspection Reports.